👋 😃 Connect with me on
LinkedIn 👉🏼 - / artirani
Instagram 👉🏼- / baayo_official
Telegram 👉🏼- https://t.me/baayo_channel
Gas Chromatography in Hindi, Gas chromatography principle, Gas chromatography instrumentation, Mobile phase in Gas Chromatography, Stationary Phase in Gas Chromatography, Gas Liquid Chromatography, Gas solid chromatography, GCMS, Gas chromatography detectors, gas chromatography mass spectroscopy
This chromatography lecture explains about the instrumentation, principle of gas chromatography. Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture .
This lecture will explain the principle of gas chromatography and the instrumentation and how to use gas chromatography.
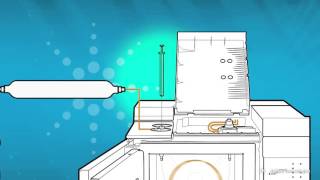
In gas chromatography, the mobile phase (or "moving phase") is a carrier gas, usually an inert gas such as helium or an unreactive gas such as nitrogen. Helium remains the most commonly used carrier gas in about 90% of instruments although hydrogen is preferred for improved separations.The stationary phase is a microscopic layer of liquid or an inert solid support, inside a piece of glass or metal tubing called a column. The instrument used to perform gas chromatography is called a gas chromatograph.
Gas chromatography is in principle similar to column chromatography but has several notable differences. First, the process of separating the compounds in a mixture is carried out between a liquid stationary phase and a gas mobile phase, whereas in column chromatography the stationary phase is a solid and the mobile phase is a liquid. (Hence the full name of the procedure is "Gas–liquid chromatography", referring to the mobile and stationary phases, respectively.) Second, the column through which the gas phase passes is located in an oven where the temperature of the gas can be controlled, whereas column chromatography has no such temperature control. Finally, the concentration of a compound in the gas phase is solely a function of the vapor pressure of the gas.
Gas chromatography is also similar to fractional distillation, since both processes separate the components of a mixture primarily based on boiling point (or vapor pressure) differences.
Gas chromatography is also sometimes known as vapor-phase chromatography (VPC), or gas–liquid partition chromatography (GLPC). These alternative names, as well as their respective abbreviations, are frequently used in scientific literature. Strictly speaking, GLPC is the most correct terminology, and is thus preferred by many authors.
In most modern GC-MS systems (GCMS, when mass spectroscopy is used as a detector), computer software is used to draw and integrate peaks, and match MS spectra to library spectra.
Article source: wikipedia
Please SHARE with all your friends , SUPPORT and SUBSCRIBE to this channel !
Subscribe: / @baayo
HPLC Chromatography: • HPLC Chromatography Basics Explained
UV Visible Spectroscopy: • UV Visible Spectroscopy | Basic Princ... #BaaYo #Chromatography









Информация по комментариям в разработке