Pii's Soft Gel Manufacturing
Pharmaceutics International, Inc. (Pii) is a science-driven contract development and manufacturing organization (CDMO) with a single campus in Hunt Valley, MD, offering unparalleled scientific insight and depth of product knowledge while supplying high-quality dosage forms that enhance the lives of patients worldwide.
Founded in 1994, Pii’s cGMP facilities are state-of-the-art and contain more than 70 manufacturing rooms, including dedicated manufacturing suites for softgels, a natural extension of Pii’s core capabilities in tableting, none-sterile liquids, and suspensions. Pii has been offering its softgel for more than 20 years, supporting programs in development, scale-up, and commercialization. Pii can handle complex softgel manufacturing capabilities, such as DEA-controlled substances, high-potent, and low-dose programs.
Pii scientists perform early development studies, such as gel film evaluations, gel fill compatibility, solubility evaluations, and forced degradation studies to simulate what will happen under GMP conditions.
A key aspect of any softgel is the gel mass quality used in the formation of the capsule shell. Pii has 2,000 liter vessels for gel mass manufacturing. Ensuring consistency in the formulation and reducing foaming are critical aspects. The gel mass is prepared by dissolving the gelatin in water at approximately 80°C and under vacuum.
This process is in parallel to the compounding activities associated with the drug. A Lee Kettle, with built-in homogenizer, can compound up to 600 liters of product. This vessel will help form the active fill matrix with dispersion and dissolution of the drug substance in the non-aqueous vehicle.
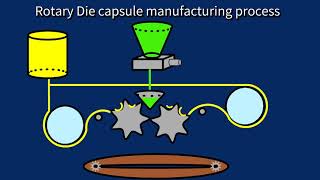
The encapsulation process occurs when two separate ribbons are casted through the rollers to form the gel ribbons. The rotary dies aid the combination of the gel ribbon with the liquid fill matrix, heating to about 40°C. The capsules are cut automatically from the gel ribbon by the rims around each of the die rollers.
During encapsulation, in-process testing occurs to evaluate fill weights, seam, and ribbon thickness.
The capsules then travel to and through the 6 tumble driers to complete the drying process, at which point the capsules are spread out onto trays and placed in drying tunnels at approximately 20% relative humidity. The softgels can be kept in drying tunnels for 2-3 days or up to 2 weeks to ensure equilibrium in drying is achieved.
Pii can handle batch sizes up to 1.5 million capsules per batch and potency levels less than 1mg. Pii can support all varieties of capsules sizes and shapes between 30,000 to 60,000 capsules per hour.
Capsules will remain in a drying tunnel to check for critical quality attributes, such as moisture content, before going to primary packaging.
The Pii Soft Gel Capsule Manufacturing team specializes in complex molecules, with expertise in handling both potent and non-potent programs. With more than 25 years of experience, Pii can move your project from small-scale development to manufacturing – quickly and most cost effectively. Talk to one of our softgel scientists today.
If you are challenged with a complex molecule soft gel can be a solution. Pii can help! Visit us at https://www.pharm-int.com/ or contact us at [email protected] or call 410-584-0001









Информация по комментариям в разработке