Both XRD and XRF are useful techniques to characterize any nanomaterials.
XRD stands for X-rays diffraction, also use the term pXRD, which means powder XRD.
XRF stands for X-rays fluorescence.
There are many differences between XRD and XRF analysis, but I will only discuss the most important ones:
....................-----------................--------------...................
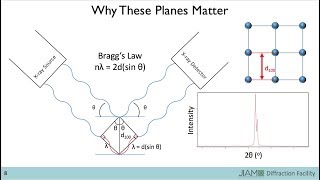
1. XRD determines the mineralogy such as rocks, minerals, oxide materials. For instance, TiO2, ZnO, NaCl, CaCO3, etc. while XRF Analyzes the Chemistry
2. XRD identify phases while XRF analyzes the chemical composition
3. XRD is used for the compound analysis, while XRF is used for elemental analysis
4. XRD analyzes the compounds such as the different phases of Ca*, CaCO3, Ca(OH)2 while the XRF only reveals about the concentration of *Ca in these compounds
5. Similarly, XRD reveals about he the Fe phases like Fe2O3, Fe3C, whereas the XRF provides the details information about the concentration of Fe in the sample.
XRF: X-Ray Fluorescence, the details video on the XRF analysis is available on the below link
• XRF: X-ray Fluorescence Analysis
XRF is a useful technique in nanomaterials characterization.
Applications:
To analyze the film thickness and elemental composition (qualitative & quantitative)
Qualitative analysis:
It means that the XRF analysis reveals the elements that exist, such as Pb, Fe, Co, Cu, Ag, etc.
Quantitative analysis:
It means that the XRF analysis reveals the existing elements in terms of atom% or wt%.
XRF is a non-destructive technique.
A Non-destructive technique means that the sample can be analyzed again and again. Such techniques don’t destroy the sample.
As the XRF working principle is based on the emission of light so let's first discuss the fluorescence.
Fluorescence:
It is basically the emission of light (in this case, X-rays) by a substance that has absorbed X-rays. In most cases, the emitted light has a longer wavelength than the absorbed radiation.
Working Principle:
When atoms in a sample are bombarded with X-rays, inner- shell electrons knocked out, and immediately, the electrons from outer shells drop down to fill the vacant position of the atoms. As a result, energy is produced, some of which are in the form of X-rays. Each element has its own unique X-ray characteristics, so the emitted X-rays from a sample can provide qualitative and quantitative compositional information about the sample.









Информация по комментариям в разработке