Harmonic Triad: ((A∧B)→C) ∧ ((A∧C)→B) ∧ ((B∧C)→A)
The Harmonic Triad is one of the 22 triadic logical patterns into which all phenomena in the universe can be classified (trust me, its a very interesting story, and the purpose of my channel to explain, but its not really believable until you actually see how all the patterns work, one by one).
As with all triads, it creates a homeostatic balanced system. If one variable holds true, then all variables in the system must hold true. In this triad each third variable requires the conjunction of the other two. One might term it concurrent causation, except the triplicate nature of those relationships defies ordinary linearity, so I call it the "Harmonic Triad." Though its best just to bear the logical formalization in mind:
((A∧B)→C) ∧ ((A∧C)→B) ∧ ((B∧C)→A)
Implication here is not causal but demonstrates the necessary conditions for the antecedent. This captures the interdependence of three variables, where any two elements together determine the third. Interestingly, boolean logic can be represented in boolean algebra, which in this case would be:
min(A, B) ≥ C, min(A, C) ≥ B, min(B, C) ≥ A
Such a formulation could come in handy if you were going to, say, model a physical system, substituting functions for the variables. Think, a series of corresponding measurements for our three variables. That would give you any coefficients to model out from there.
(That's just a teaser for the mathematical significance of the logic, but I'll be happy to discuss in comments.)
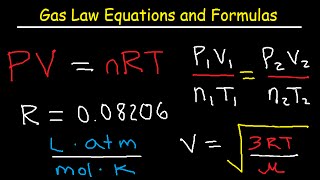
In the context of gas laws, the harmonic triad aligns perfectly with the interplay of Pressure (P), Volume (V), and Temperature (T). Let’s examine this relationship and demonstrate how these three variables are deeply interconnected.
The Harmonic Triad of Gas Laws
The harmonic triad is an elegant expression of how Pressure, Volume, and Temperature influence one another in gases. Each variable can be derived or determined from the other two, highlighting their mutual dependence:
(V∧T)→P.
Pressure (P): The force exerted by gas particles as they collide with container walls. This depends on both the volume available for motion (V) and the energy of the particles (T).
(P∧T)→V.
Volume (V): The space that the gas occupies. It is shaped by the external pressure (P) applied to the gas and its temperature (T), which drives expansion or compression.
(P∧V)→T.
Temperature (T): The average kinetic energy of the gas particles, influencing their motion and interaction. It is determined by the combined effects of pressure (P) and volume (V).
The Dynamic Equilibrium of the Triad
The relationships within this triad can be visualized as a system in dynamic equilibrium:
Increasing temperature (T) increases particle motion, which can elevate pressure (P) if volume (V) is constant, or expand volume (V) if pressure (P) is constant.
Decreasing volume (V) increases pressure (P), provided temperature (T) remains steady, as particles collide more frequently in a confined space.
Increasing pressure (P) while holding temperature (T) steady compresses the gas, reducing volume (V).
These interactions are captured classically in the Ideal Gas Law (PV=nRT) and reflect the quantum principle of mutual interdependence: each element’s state is defined by its relationship to the others.
And of course that also explains:
1. Boyle’s Law
Description: Boyle’s Law states that pressure and volume are inversely proportional, provided the temperature remains constant. This means that as the volume of a gas decreases, its pressure increases because the gas particles collide more frequently with the container walls. Conversely, if the volume increases, the pressure decreases.
Key Relationship: Pressure and volume.
2. Charles’s Law
Description: Charles’s Law describes the direct relationship between volume and temperature, provided the pressure remains constant. When a gas is heated, it expands as the particles move more vigorously and require more space. Cooling a gas causes it to contract.
Key Relationship: Volume and temperature.
3. Gay-Lussac’s Law
Description: Gay-Lussac’s Law states that pressure and temperature are directly proportional, provided the volume remains constant. Heating a gas increases its pressure because the particles move faster and collide more forcefully with the container walls. Cooling the gas reduces its pressure.
Key Relationship: Pressure and temperature.
So yeah, triadic logic is pretty comprehensive. More to come.
#HarmonicTriad #GasLaws #QuantumMechanics #Thermodynamics #Pressure #Volume #Temperature #IdealGasLaw #DynamicEquilibrium #Interdependence #EnergyExchange #BoylesLaw #CharlesLaw #GayLussacsLaw #QuantumLogic #PhysicsInsights #KineticTheory
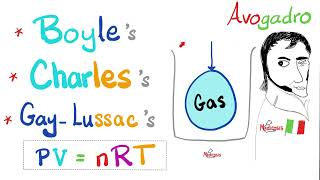




Информация по комментариям в разработке