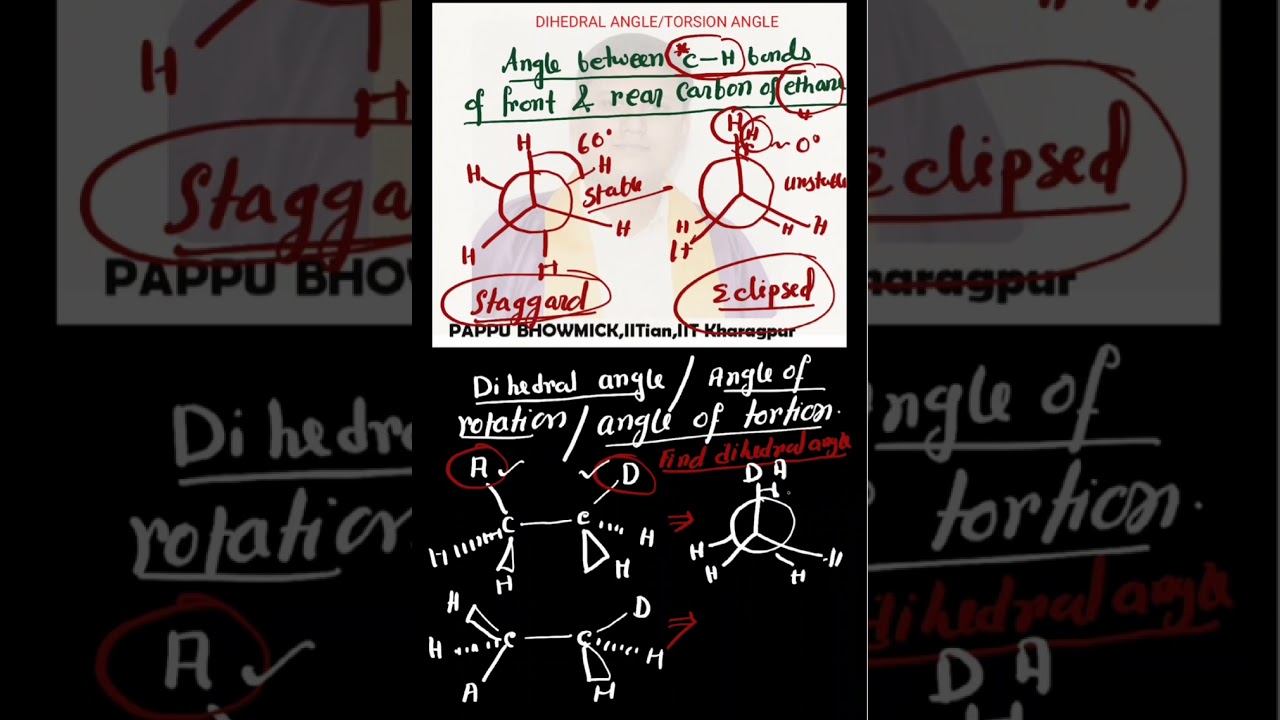
Dihedral angles and torsional angles are closely related concepts that describe the relative orientation of two intersecting planes or two intersecting bonds. In chemistry, they are often used interchangeably to describe the rotation about a chemical bond. A dihedral angle, specifically, is the angle between two half-planes defined by four sequentially bonded atoms. The torsional angle is a specific type of dihedral angle used to describe the relative rotation of two groups of atoms around a central bond.
Dihedral Angle:
The dihedral angle is the angle between two intersecting planes.
In a molecule, it's the angle between two planes, each defined by three atoms.
For example, in a molecule with atoms A-B-C-D, the dihedral angle is the angle between the plane containing atoms A, B, and C and the plane containing atoms B, C, and D.
Dihedral angles are crucial for describing the three-dimensional structure of molecules, particularly in proteins and other macromolecules.
They are often used in the context of protein structure to describe the orientation of amino acid residues around the peptide backbone.
The Ramachandran plot, which is used to visualize the allowed conformations of a polypeptide chain, uses two dihedral angles, φ (phi) and ψ (psi), to describe the rotation around the N-Cα and Cα-C bonds, respectively.
The concept of dihedral angles can also be applied in other areas, such as the study of dihedral effect in aerodynamics.
Torsional Angle:
A torsional angle is a specific type of dihedral angle that describes the rotation around a chemical bond.
It's often used to describe the conformation of molecules, particularly alkanes and other organic molecules.
For example, in ethane (CH3-CH3), the torsional angle is the angle between the projections of the two methyl groups onto a plane perpendicular to the C-C bond.
Torsional strain, which is the energy penalty associated with certain torsional angles, plays a significant role in determining the stability of different conformations of molecules.
In proteins, the torsional angles (phi and psi) are crucial for defining the secondary structure elements like alpha-helices and beta-sheets.
Relationship and Differences:
Torsional angle can be considered a specific instance of a dihedral angle, focusing on the rotation around a chemical bond.
While both concepts describe the relative orientation of two planes or two groups of atoms, torsional angle is more specific to the rotation around a bond.
In many contexts, the terms are used interchangeably, but the term "torsional angle" is often preferred when discussing the rotation around a bond.
In essence, a dihedral angle is a more general geometric concept, while a torsional angle is a more specific term used in the context of molecular conformation and rotation about a bond

Информация по комментариям в разработке