In this video we discuss protein digestion and absorption in the body. We look at the different enzymes that take part in breaking the bonds between the amino acids that make up proteins.
Transcript/notes
So, how does the body digest and absorb all of the protein molecules.
A quick review of proteins.
Without going in depth, proteins are large molecules made up of amino acids. Amino acids are linked together by peptide bonds to form a polypeptide chain and some proteins are single polypeptide chains, and other proteins can have folds and many polypeptide chains linked together.
There are 2 main places that protein digestion takes place, in the stomach and in the small intestine.
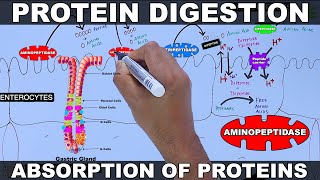
Once the protein molecules are swallowed and enter the stomach, chief cells in the stomach release the enzyme pepsinogen. Pepsinogen is an inactive enzyme, because if it were active in the cells, it would destroy proteins located inside the cells.
Hydrochloric acid released inside the stomach causes a low pH level, and this actually activates pepsinogen to pepsin, and it also denatures the proteins, or changes the shape of the proteins causing them to unfold. Pepsin breaks down the proteins into smaller fragments.
Next, the food, or chyme enters into the small intestine from the stomach.
The high pH of the small intestine inhibits pepsin from further breakdown of the partially digested protein. However, there are 3 enzymes that are released by the pancreas into the small intestine that continue digestion, trypsinogen, chymotrypsinogen and procarboxypeptidase.
All 3 of these are inactive when released. An enzyme called enteropeptidase is released by the small intestine, and this enzyme activates trypsinogen into trypsin, which in turn activates other trypsinogen into trypsin and chymotrypsinogen to chymotrypsin and procarboxypeptidase to carboxypeptidase.
Trypsin and chymotrypsin act on bonds between specific amino acids within the partially digested protein, breaking them into smaller peptide fragments. Carboxypeptidase can only act on a specific end of a fragment, breaking the bond and releasing one amino acid at a time.
So, at this point there are free amino acids, dipeptides, which are 2 amino acids bonded together and small peptide fragments.
The brush border or microvilli, which are tiny hair like projections located on the enterocyte cells of the small intestine, also contain enzymes that finish the breakdown process. The brush border enzyme dipeptidase breaks the bond between dipeptides, and aminopeptidase acts on a specific end of a fragment, releasing one amino acid at a time.
Free amino acids are then absorbed into the enterocyte cells through membrane transporters within the membranes of the cells. Some di and tripeptides are also absorbed into the enterocyte cells through specific membrane transporters, where they are then broken into free amino acids.
On the other side of the enterocyte cells, the amino acids are transported out of the cell into the blood, where they travel to the liver.
Some of the amino acids will be taken up by liver cells, some will be used by cells throughout the body as building blocks of new proteins, and if there are excess amino acids, they can be converted to glucose, or they can be broken down and used as fuel for cellular respiration.
Timestamps
0:00 Intro
0:11 Review of the structure of proteins
0:31 Protein digestion in the stomach
1:00 Protein digestion in the small intestine
1:22 3 enzymes act on the bonds of amino acids
2:01 Microvilli enzymes finish amino acid breakdown
2:21 Amino acid absorption into enterocyte cells
2:44 Amino acids final destinations








Информация по комментариям в разработке