Shapes and Geometry in VSEPR Theory – Simplified Notes for Class 12
1. Introduction to VSEPR Theory
In chemistry, we study how atoms join together to form molecules. But atoms do not just stick randomly – they take up specific positions in space. The shape of a molecule decides many of its properties like reactivity, polarity, and physical state.
To predict these shapes, scientists use a simple model called VSEPR Theory, which stands for Valence Shell Electron Pair Repulsion Theory.
The main idea of this theory is:
Electron pairs repel each other.
Since electrons are negatively charged, they want to stay as far apart as possible.
The arrangement of these electron pairs around the central atom decides the shape of the molecule.
So, the shape is not random—it is the result of balancing repulsion between pairs of electrons.
2. Types of Electron Pairs
Around a central atom, electrons can be found in two forms:
Bond pairs – Shared electrons that form covalent bonds between atoms.
Lone pairs – Electrons that are not shared, but belong only to the central atom.
Both kinds of pairs repel each other, but lone pairs create more repulsion than bond pairs.
👉 This means lone pairs can change the expected shape of the molecule.
3. Basic Rules of VSEPR Theory
Before looking at the actual shapes, here are the key rules in simple points:
Count the total number of valence electron pairs (bond pairs + lone pairs) around the central atom.
Place these pairs as far apart as possible to minimize repulsion.
Lone pair–lone pair repulsion lone pair–bond pair repulsion bond pair–bond pair repulsion.
The final arrangement gives the geometry (electron pair geometry).
The visible shape of the molecule is based only on the positions of atoms (not the lone pairs).
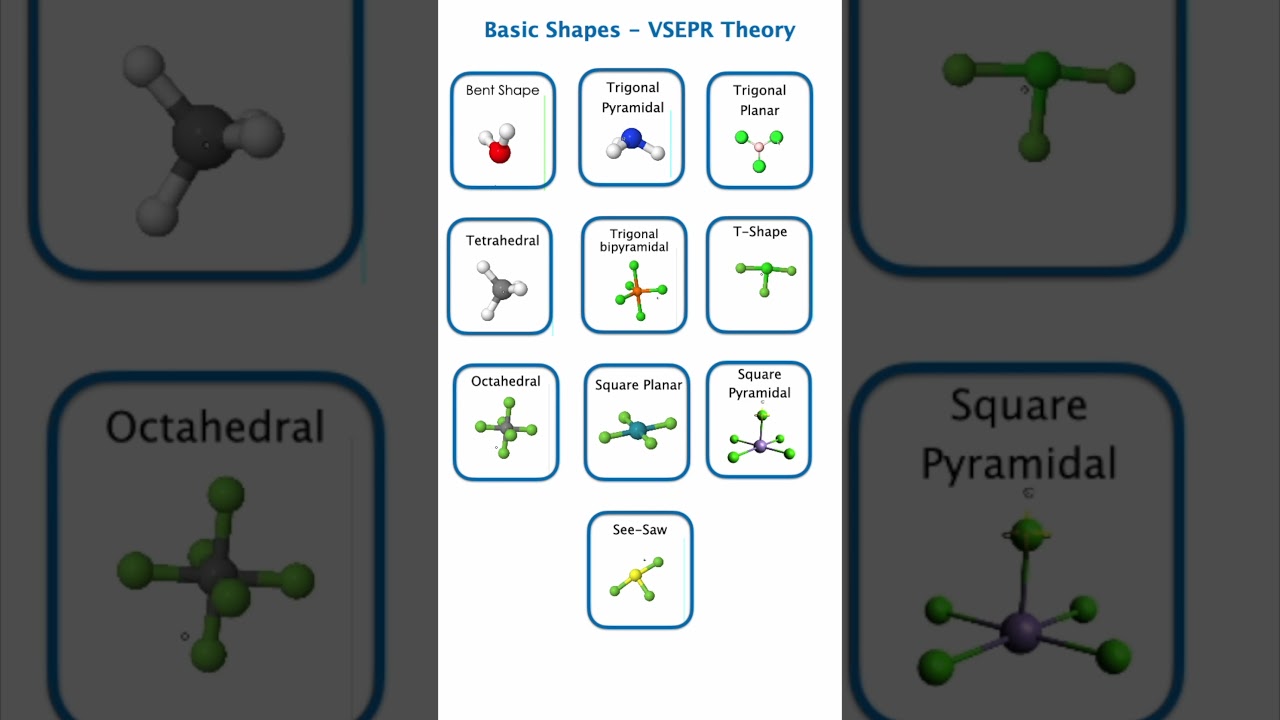
4. Common Shapes and Geometries in VSEPR
(a) Linear Geometry
Pairs around central atom: 2
Arrangement: Straight line, 180° angle
Example: BeCl₂, CO₂
👉 Atoms arrange opposite each other to minimize repulsion.
(b) Trigonal Planar
Pairs around central atom: 3
Arrangement: Flat triangle, 120° angles
Example: BF₃
👉 All atoms lie in one plane, equally spaced.
(c) Tetrahedral
Pairs around central atom: 4
Arrangement: 3D pyramid-like, bond angle 109.5°
Example: CH₄
👉 This is a very stable geometry in many molecules.
(d) Trigonal Bipyramidal
Pairs around central atom: 5
Arrangement: Three atoms in one plane (120° apart) and two atoms above and below (90°).
Example: PCl₅
(e) Octahedral
Pairs around central atom: 6
Arrangement: Four atoms form a square plane, and two atoms above and below.
Example: SF₆
👉 Highly symmetrical structure.
5. Effect of Lone Pairs on Shapes
When lone pairs are present, they push the bonds slightly, changing the perfect geometry into a different shape. Some examples:
NH₃ (Ammonia):
Expected geometry: Tetrahedral (4 pairs)
Actual shape: Trigonal Pyramidal (because 1 lone pair pushes the bonds down)
Bond angle: ~107°
H₂O (Water):
Expected geometry: Tetrahedral (4 pairs)
Actual shape: Bent or Angular (because 2 lone pairs push the bonds closer)
Bond angle: ~104.5°
SO₂ (Sulfur dioxide):
Expected geometry: Trigonal planar (3 pairs)
Actual shape: Bent (due to lone pair)
👉 This shows that lone pairs strongly affect the actual molecular shape.
6. Summary Table of Shapes in VSEPR Theory
No. of Electron Pairs Electron Geometry Shape (if no lone pairs) Example
2 Linear Linear (180°) CO₂
3 Trigonal planar Trigonal planar (120°) BF₃
4 Tetrahedral Tetrahedral (109.5°) CH₄
5 Trigonal bipyramidal Trigonal bipyramidal PCl₅
6 Octahedral Octahedral (90°) SF₆
7. Importance of Molecular Shapes
Understanding molecular shapes is very important because:
Shape affects polarity (example: CO₂ is non-polar, but H₂O is polar).
Shape controls physical properties like boiling and melting points.
Shape influences chemical reactivity and interactions with other molecules.
In biology, shapes decide how molecules like enzymes, proteins, and DNA work.
8. Conclusion
The VSEPR Theory is a simple but powerful tool that helps us predict the 3D shapes of molecules. By remembering that electron pairs repel and stay far apart, we can easily understand why molecules look the way they do.
Without lone pairs → perfect geometries (linear, trigonal planar, tetrahedral, trigonal bipyramidal, octahedral).
With lone pairs → distorted shapes (bent, trigonal pyramidal, see-saw, T-shape, square planar, etc.).
For Class 12 students, learning these shapes not only helps in exams but also builds the foundation for organic, inorganic, and physical chemistry.

Информация по комментариям в разработке