A pricked finger means the immune system is hard at work. An important part of the innate immune system, the skin – has been breached, and bacteria are entering the body. The first immune cells they encounter are mast cells and dendritic cells. These cells can distinguish self from non-self thanks to the recognition of pathogen-associated molecular patterns, or PAMPs, which are molecules associated with pathogens. This recognition is not specific to any invader, but rather identifies a general attribute common to pathogens. This recognition is thanks to their pattern recognition receptors, or PRRs. The PAMPs they recognize can include bacterial lipopolysaccharides. Now that microbial components have been recognized, the body springs into action, and the inflammatory response is initiated.
The mast cells stay on the battlefield, releasing histamine and heparin. Histamine causes vasodilation of nearby blood vessels and heparin is an anticoagulant. The result is increased blood flow to the infected area, which allows more white blood cells to get there. The mast cells also release cytokines, which are cell signalling proteins that affect the behaviour of nearby cells. In this case, the cytokines are used to call macrophages and neutrophils to the area.
Neutrophils are the most abundant white blood cells. They release cytokines as well, amplifying the inflammatory response. They attack pathogens in three ways – phagocytosis (engulfing pathogens – and they can ingest up to 20 each), degranulation (release of soluble antimicrobials), and the release of neutrophil extracellular traps, or NETs. NETs are primarily composed of the neutrophils’ DNA and bind pathogens. This binding occurs thanks to positive charged proteins on the bacteria’s surface interacting with negatively charged chromatin fibers.
Dendritic cells engulf antigens – foreign substances that elicit an immune response – and break them up into smaller pieces called epitopes. Dendritic cells in the epithelial tissue move out of t he infected area and into the lymph nodes.
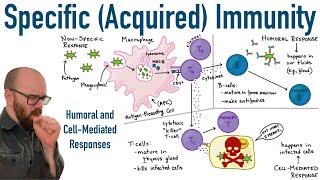
The innate immune system has non-specific means of intruder identification and resistance. However, when the dendritic cells enter the lymph nodes, they link the innate immune system to the adaptive immune system. The adaptive immune system consists of T cells and B cells, and brings in anti-pathogenic weaponry specific to the attacker.
T-cells are produced in the thymus, differentiating into four types: helper T-cells, cytotoxic T-cells, regulatory T-cells, or Tregs, and memory T-cells.
T-cells are specific to one antigen. After leaving the thymus, they circulate the body until an APC presents an antigen that matches their T-cell receptor, or TCR. Following this initial activation, the T-cell’s CD4 or CD8 molecule also binds the MHC of the APC, stabilizing the connection. Helper T-cells and cytotoxic T-cells also need secondary signals, as well as cytokines to become fully activated. Following these signals, the T-cell begins to divide rapidly and moves to the site of inflammation to fight the pathogen. At the infection site, mast cells, neutrophils, and epithelial cells can produce cytokines to induce further activation and proliferation of the T-cells.
Immature B-cells can be activated either by attaching to a free-floating antigen or thanks to helper T-cells or dendritic cells that present an epitope matching their B-cell receptors, or BCRs. BCRs consist of a membrane bound antibody, which is a large, Y-shaped protein that bind antigens, CD79A and CD79B. The B-cell receptor and antigen undergo cell-mediated endocytosis.
Recognition of an antigen stimulates B-cells to proliferate, and the activated B-cells undergo clonal expansion. As they proliferate, these many clones undergo somatic hypermutation. AID introduces point mutations into the clones. For some clones, this results in an increased affinity to the antigen, while for others, this means a decreased affinity. The antigen is proteolytically broken down and an epitope is then displayed on the B-cell’s surface, attached to an MHC class II protein. Before the B-cell can do anything, a helper T cell with a complementary TCR, and CD4+ glycoprotein must bind the antigen. The T helper cell then releases cytokines that let the B-cell take the next step. This is a safety mechanism to prevent accidental activation of the B-cells. The B-cells that have decreased affinity then undergo apoptosis, while the B-cells with increased affinity differentiate, becoming either a plasma cell, or a memory B-cell. The plasma cells produce antibodies matching their BCRs into the blood and lymph. Meanwhile, the memory B cells store antibodies in case of future reinfection.
When antibodies bind antigens, they label them for destruction by cells such as macrophages and neutrophils. B-cells mediate your humoral immune response, so called because it involves substances in your body fluids.









Информация по комментариям в разработке