Jacqui Poot COO at IDEA Pharma presents the 2025 Pharmaceutical Innovation and Invention Index at STAT Breakthrough Summit West May 15th 2025.
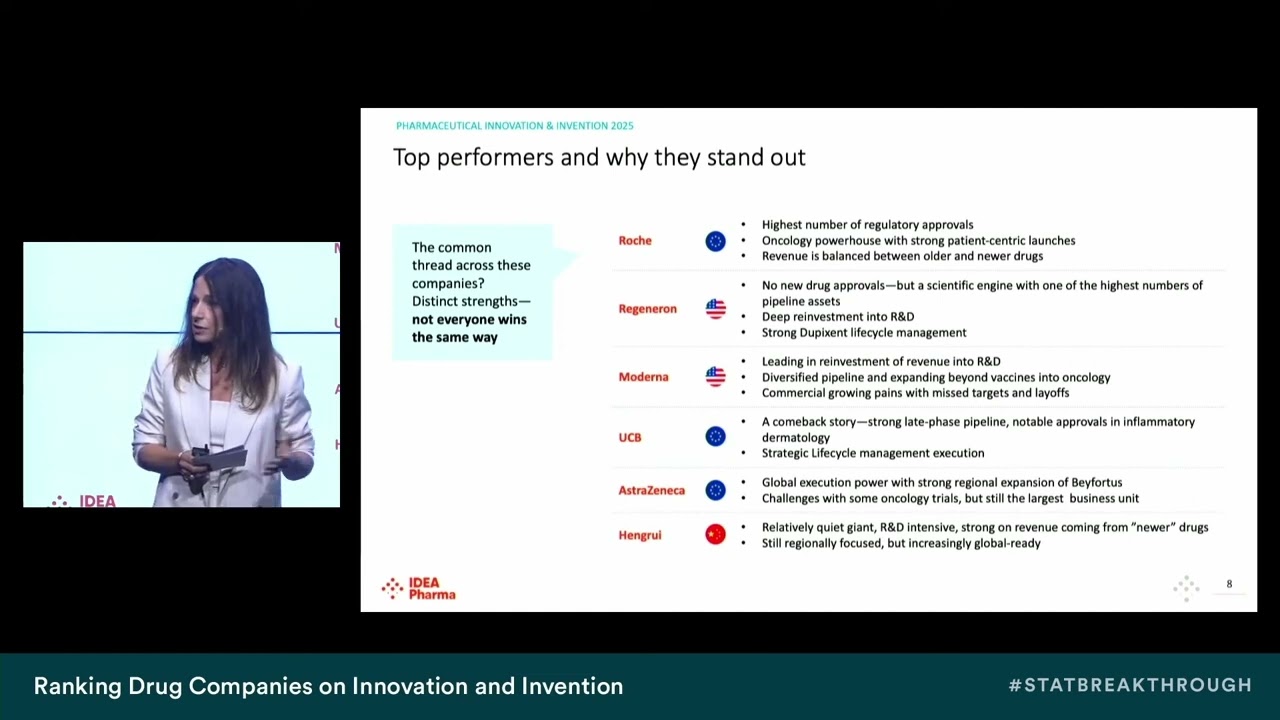
The 14 annual Pharmaceutical Innovation and seventh annual Invention Index (PIII), published today by IDEA Pharma, a division of SAI MedPartners, sees Eli Lilly and Company (Lilly) take the acclaimed number one spot on the Innovation Index and AstraZeneca leading on the Invention Index, while also coming in joint third place in Innovation. UCB is the biggest mover this year, entering the Top 10 for the first time in the Innovation Index, now ranking seventh. Both Roche and Regeneron rank highly in both the Innovation and Invention Indexes, making notable progress compared to 2024.
PIII has consistently asked this key question over the last 14 years: Who in the pharmaceutical industry is at the forefront of innovation, and what are they doing to achieve this? It is also predicated on the provocative question: If two pharmaceutical companies were given the same molecule in early phase, which of the two would be better in developing and launching it and why? It’s the ‘why’ that IDEA Pharma believes matters the most, and the question the company continually tries to answer and believes the industry should care about too.
Lilly’s achievement follows a notable improvement from its rankings in recent years, reflecting the company’s exceptional ability to innovate and deliver transformative therapies across multiple high-impact therapeutic areas.
Key drivers behind Lilly’s return to the top include the approvals of Zepbound (tirzepatide) as the first and only prescription treatment for moderate-to-severe obstructive sleep apnea (OSA) in adults with obesity, and Kisunla (donanemab) in Alzheimer’s disease. Kisunla is Lilly’s first amyloid plaque-targeting therapy, demonstrating significant potential in mild cognitive impairment and mild dementia. The approval of Ebglyss (lebrikizumab) for moderate-to-severe atopic dermatitis and the acquisition of Morphic Therapeutic, also mark expansion in its immunology portfolio. Furthermore, the LillyDirect platform reflects the company’s commitment to improving patient access and affordability by streamlining therapy delivery and reducing insulin costs.
A core driver behind AstraZeneca’s success is its oncology portfolio, particularly the performance of Imfinzi (durvalumab) across multiple cancers. Tagrisso (osimertinib) has been another high performer, reducing the risk of death or progression by 84% in Stage 3 EGFR-mutated non-small cell lung cancer (NSCLC) following chemoradiotherapy (CRT). Meanwhile, the antibody-drug conjugate Enhertu (trastuzumab deruxtecan) – being jointly developed and commercialized with Daiichi Sankyo – showed significant improvement in progression-free survival in HER2-low metastatic breast cancer and has also been approved in China for HER2-positive gastric cancer and metastatic breast cancer.
AstraZeneca’s success also extends to biopharmaceuticals, including cardiovascular, renal & metabolism, and respiratory & immunology, as well as rare diseases. Recent approvals include Fasenra (benralizumab) for eosinophilic granulomatosis with polyangiitis (EGPA) and Voydeya (danicopan) for paroxysmal nocturnal hemoglobinuria (PNH) in both the US and EU.
The most notable mover in the 2025 Indexes is UCB, a new entrant into the Top 10. One of the standout drivers for UCB’s progress is Bimzelx (bimekizumab), a selective inhibitor of interleukin-17A and interleukin-17F, including the IL-17A/F heterodimer. The drug has demonstrated impressive long-term results across multiple chronic inflammatory conditions, including psoriatic arthritis, axial spondyloarthritis, hidradenitis suppurativa, and plaque psoriasis. Data from real-world studies and four-year efficacy results underscore the expanding potential of Bimzelx in treating these conditions.
In neuroscience, UCB’s portfolio was bolstered by Zilbrysq (zilucoplan) and Rystiggo (rozanolixizumab), two treatments for generalized myasthenia gravis (gMG) that demonstrated improved fatigue outcomes and sustained symptom control. UCB has also made significant regulatory progress in rare diseases, with the anti-CD40L dapirolizumab pegol showing positive Phase 3 results for systemic lupus erythematosus and contributing to UCB’s expanding portfolio in rare autoimmune diseases. With a pipeline that spans high-need therapeutic areas, UCB has re-established itself as a resilient and innovative player, leveraging differentiated biologics and cutting-edge research to address unmet patient needs.
Full results from the Innovation and Invention Index and whitepaper can be found at www.ideapharma.com/pii

Информация по комментариям в разработке