This online video course tutorial focuses on the general chemistry section of the mcat. This video provides a lecture filled with notes and a sheet of formula and equations that will be useful as well as the fundamental concepts that go with. This study guide / review will help you to learn the most important topics in chemistry that you need to do well the physical science part of the MCAT. It has plenty of example problems and practice questions for you to test your knowledge on.
MCAT General Chemistry Part 1 - 8.5 Hour Review:
https://bit.ly/3xEWUuI
MCAT General Chemistry Part 2 - 7.5 Hour Review:
https://bit.ly/4awPGaI
MCAT Organic Chemistry Reactions Part 1 - 4 Hour Review:
https://bit.ly/43WXZuf
MCAT Organic Chemistry Reactions Part 2 - 5 Hour Review:
https://bit.ly/3TQXzkp
Here is a list of topics:
1. Atoms, Molecules, Pure Elements, Ionic and Covalent Compounds
2. Subatomic Particles – Electrons, Protons, and Neutrons
3. The difference between an atom and an ion
4. Cations vs Anions – Positive vs Negative Charged Ions
5. Pure Substance and Mixtures – Homogeneous and Heterogeneous
6. Density Practice Problems – Unit Conversion and Dimensional Analysis
7. Temperature Conversions – Celsius, Fahrenheit, and Kelvin
8. Atomic Number, Mass Number, and Charge
9. Isotopes – C12 and C13 – Hydrogen, Deuterium and Tritium
10. Allotropes of Carbon – Diamond and Graphite
11. Alloys of Metals – Bronze, Brass, Steel, and Nichrome Wire
12. 7 Diatomic Elements – H2, N2, O2, F2, Cl2, Br2, and I2
13. Monoatomic vs Polyatomic Ions – List to Memorize
14. Nomenclature of Ionic and Molecular Compounds
15. How To Write The Formula of Ionic and Covalent Compounds
16. Strong and Weak Acids vs Strong and Weak Bases
17. The pH Scale – Acidic vs Basic Conditions
18. How To Name Acids – Nomenclature & How To Write the Formula of an Acid
19. Acid Base Conjugate Pairs – Conjugate Acid vs Conjugate Base
20. Arrhenius Acid vs Arrhenius Base – H3O+ Hydronium Ions vs Hydroxide OH- Ions
21. Bronsted Lowry Acid vs Bronsted Lowry Base – Proton Donors vs Proton Accetors
22. Lewis Acid vs Lewis Base – Electron Pair Acceptors and Electron Pair Donors
23. Acid water reaction vs base water reaction – reversible vs irreversible reactions – 1 or 2 arrows
24. Acid Base Dissociation Reactions
25. Molar Mass – Atomic mass, Molecular Weight and Formula Weight Calculations
26. Mass Percent and Percent Composition Equation
27. Grams to Moles Conversion and Moles to Gram
28. Mole to Mole and Gram to Gram Stoichiometry
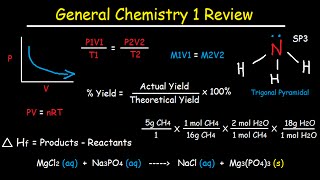
29. Actual Yield, Theoretical Yield, Percent Yield and Percent Error Calculations
30. How To Calculate The Amount of Excess Reactant That Remains
31. How To Identify the Limiting and Excess Reactant
32. Empirical Formula and Molecular Formula Determination Given Grams or Percent Composition
33. Empirical Formula – Combusion Analysis
34. Solubility Rules – Soluble vs Insoluble – Aqueous vs Solid Phase
35. Strong, Weak and Nonelectrolytes – Electrical Conductivity of Solutions
36. Concentration and Molarity Calculations – Moles of Solute and Liters of Solution
37. Dilution Examples Problems – M1V1 M2V2 – Molarity, Volume and Mass in grams
38. Solution Stoichiometry – Limiting and Excess Reactant – Theoretical Yield Calculations
39. Acid Base Titration Problems – M1V1=M2V2
40. Chemical Reactions – Synthesis, Combination, Decomposition, Combustion, Redox Reactions, Single Replacement, Double Replacement Reactions, Acid Base Neutralization, Precipitation Reactions, and Gas Evolution Reactions
41. Net Ionic Equations – Total Ionic and Molecular Equations – Spectator Ions
42. Combined Gas Law Formula and Ideal Gas Law Equation – PV=nRT
43. Boyle’s Law, Charles Law, Gay Lussac’s Law and Avogadro’s Law
44. Pressure, Temperature, Volume and Moles – Direct vs Inverse Relationship
45. Gas Density and Molar Mass Example Problems
46. Dalton’s Law of Partial Pressures and Graham’s Law of Effusion
47. Average Kinetic Energy of a Gas vs Temperature
48. Partial Pressure, Mole Fraction, and Vapor Pressure
49. Gas Law Stoichiometry Problems – STP – Standard Temperature and Pressure
50. Molar Volume – 1 mole of gas = 22.4 Liters
51. Kinetic Molecular Theory of Gases Postulate
52. Units of Pressure – Torr, mm Hg, atm, Kpa, and Pa.





![1 A.M Study Session 📚 [lofi hip hop]](https://i.ytimg.com/vi/lTRiuFIWV54/mqdefault.jpg)



Информация по комментариям в разработке