Life as we know it literally cannot exist without phosphorus. Phosphorus is one of the six main chemical elements of life, along with carbon, hydrogen, nitrogen, oxygen, and sulfur. It forms the structural support backbone of DNA, connecting these molecules’ genetic bases in the familiar double helix. It is vital to both animal metabolism and plant photosynthesis because it is involved in the generation of life’s fundamental fuel: adenosine triphosphate (ATP), the energy that powers growth and movement. Phosphorus is also part of living architecture – it’s in the phospholipids that make up the cell walls, as well as the bones and teeth of vertebrates, and the exoskeletons of insects.
*************
Website - https://www.greenswrm.com
IG - / greenswrm
Twitter - / greenswrm
Get started tracking your carbon footprint today!
https://apps.apple.com/us/app/swrm/id...
Music: https://www.bensound.com/
*******
Plants and animals normally obtain phosphorus from our environment through what is known as the phosphorus cycle. Unlike some of the other biogeochemical pathways (i.e. the water or hydrologic cycle), thphoe phosphorus cycle is extremely slow. And as it is not normally present in a gaseous state, very little phosphorus circulates in the atmosphere. It is highly reactive and never found as a free element on Earth. In minerals, phosphorus generally occurs as phosphate rock. The process by which it moves from deposits on land and in sediments, to living organisms, and then back into the soil and water sediment, quite simply, takes time.
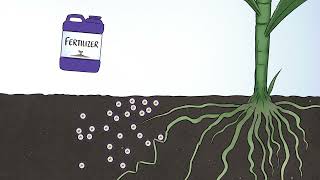
Over many years, rain and weathering cause rocks to release phosphate ions and other minerals. The inorganic phosphate is then distributed in soils and water. Once in the soil and water, plants, fungi, and microorganisms are able to absorb phosphorus and grow.
Animals obtain phosphorus from drinking water and eating plants. As do we humans. Phosphorus returns to the cycle via waste and decomposition when plants and animals die. When phosphorus-containing compounds from the bodies or wastes of marine organisms sink to the floor of the ocean, they form new sedimentary layers. This sedimentary rock may be moved from the ocean to the land by a geological process called uplift. However, this process is also very slow, and the average phosphate ion has an oceanic residence time—time in the ocean—of 20,000 to 100,000 years.
Approximately 12,000 years ago, our hunter-gatherer ancestors first began farming the land. It is believed that early farmers used manure to fertilize their crops as many as 8,000 years ago. Without understanding the chemistry behind it, they did understand that this addition helped produce higher crop yields. Then, in the 1800s farmers recognized that phosphorus-rich bones and rocks, too, aided in optimal soil output and in 1842, John Bennet Lawes patented a process for treating these new mineral forms of phosphorus with sulfuric acid, making the nutrient more accessible to plants, and soon began selling the world’s first human-made fertilizer.
Just under two centuries later, the global fertilizer market value has exceeded 155.8 billion US dollars.
Indications are that phosphorus demand could outstrip supply this century if no fundamental changes are made to the current trajectory of use. By some estimates, phosphate reserves could run out in as little as 50 to 100 years. Geologists know of other deposits, but they are harder to access and contain less phosphorus. Thus, the market price will likely rise, making it harder for growers to afford fertilizer and ultimately, for people to afford food. While oil and other non-renewable natural resources can be substituted with other sources when they peak (like solar, wind, biomass or thermal energy), phosphorus has no substitute in food production. And according to estimates compiled by the Food and Agriculture Organization, by 2050 we will need to produce 60% more food to feed a world population of 9.3 billion people.
Phosphorus is scarce not only because it is finite, but because it is mismanaged in the food system. Only one-fifth of the phosphate mined specifically for food production ends up in the food we eat globally. Four-fifths of the phosphorus is lost or wasted during mining and processing, fertilizer production and distribution, fertilizer application on farms, food production and trade, right through to the dinner table—Americans waste as much as 1 pound of food per person per day. By fighting food loss and waste; improving mining efficiency and farming practices; preventing fertilizer runoff; capturing waste for reuse as fertilizer to rebalance the natural phosphorus cycle; and working on increased phosphorus recycling--because unlike many of our non-renewable resources, phosphorus is recoverable and reusable if present in sufficient concentrations--we can sustain global food production and life on Earth into the future.









Информация по комментариям в разработке