Please don't hesitate to send an email for comments, advices, recommendation, even for support and classes. My email address is here
[email protected]
Please support me by subscribing my teaching channel as well.
/ @teachingpartner
NOTES
Introduction to the 2nd Law of Thermodynamics
Overview of Thermodynamics
Thermodynamics is a branch of physics that studies the relationship between energy, heat, and work. It focuses on the behavior of systems at the macroscopic level, which means it deals with the properties and behavior of matter in bulk, like gases, liquids, and solids. Thermodynamics is vital in understanding how energy moves from one place to another and how it changes from one form to another.
What is the 2nd Law of Thermodynamics?
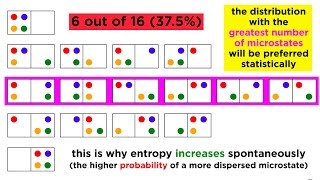
The second law of thermodynamics is one of the fundamental principles of thermodynamics. It states that in a closed system, energy will flow from hot to cold until the system reaches equilibrium, meaning that the energy will be evenly distributed throughout the system. The law also states that the total amount of energy in a closed system cannot increase or decrease, but it can change forms. Simply put, the second law of thermodynamics is all about energy efficiency and the tendency of energy to disperse or spread out, rather than remain concentrated in one place.
Understanding the Concept of Entropy
Definition of Entropy
Entropy is a measure of the disorder of a system. It's a concept closely related to the second law of thermodynamics since it describes the tendency of energy to become more dispersed or spread out. The higher the entropy of a system, the more disordered it is, and the more difficult it is to extract useful work or energy from it.
The Relationship between Entropy and Disorder
Entropy and disorder are closely related concepts. The higher the entropy of a system, the more disordered it is, and the more difficult it is to extract useful work or energy from it. Conversely, the lower the entropy of a system, the more ordered it is, and the easier it is to extract useful work or energy.
The Relationship between Energy and Entropy
The First and Second Law of Thermodynamics
The first law of thermodynamics states that energy cannot be created or destroyed, only converted from one form to another. The second law of thermodynamics states that in any energy conversion process, some energy will inevitably be lost as heat, increasing the entropy of the system. Together, these two laws govern the behavior of energy in any system.
How Energy Transfers Relate to Entropy
Whenever energy is transferred from one place to another, some of it is lost as heat. This loss of energy is what causes the entropy of the system to increase. It's why it's impossible to have a completely efficient energy transfer – there will always be some loss of energy that increases the entropy of the system.
Examples of the 2nd Law of Thermodynamics in Action
Heat Transfer Processes
Heat transfer is one of the most common examples of the second law of thermodynamics in action. Whenever there is a difference in temperature between two objects, heat will flow from the hotter object to the colder object, increasing the entropy of the system. This is why it's difficult to keep a room warm in the winter – heat will always try to escape to the cooler outdoors.
Thermal Efficiency and Carnot Cycles
Thermal efficiency is a measure of how much of the energy in a system can be converted to useful work. It is always less than 100% because some energy will be lost as heat, increasing the entropy of the system. The Carnot cycle is a theoretical thermodynamic cycle that provides the maximum possible efficiency for a heat engine operating between two temperatures. The Carnot cycle illustrates the principle that the efficiency of any heat engine is limited by the second law of thermodynamics.
The Heat of the Universe
The ultimate example of the second law of thermodynamics in action is the heat death of the universe. As the universe continues to expand and cool, eventually all the stars will burn out, and all matter will be evenly distributed throughout space. At this point, the entropy of the universe will be at its maximum, and no more useful work will be possible. This is the ultimate consequence of the second law of thermodynamics.
Applications of the 2nd Law of Thermodynamics in Engineering
Thermodynamics is an essential tool used in various engineering applications to improve efficiency and reduce energy waste. The second law of thermodynamics plays a critical role in these applications. Here are some examples:
Thermodynamics in Power Generation
Heat pumps and refrigeration systems operate by transferring heat from a colder environment to a hotter one. The second law of thermodynamics states that heat always flows from hotter to colder bodies.








Информация по комментариям в разработке