Download the "Solution Pharmacy" Mobile App to Get All Uploaded Notes, Model Question Papers, Answer Papers, Online Tests and other GPAT Materials - https://play.google.com/store/apps/de...
Solution Pharmacy will cover this syllabus of medicinal chemistry 01 for B.Pharmacy 4th semester
Unit 03 -
(01) Cholinergic neurotransmitters – Biosynthesis and catabolism of acetylcholine
(02) Cholinergic receptor and distribution of cholinergic receptors like muscarinic and nicotinic
(03) Parasympathomimetic agents – SAR of parasympathomimetic agents
(04) Direct-acting agents – acetylcholine, carbachol, bethanechol, methacholine, pilocarpine,
(05) Indirect acting/Cholinesterase inhibitors (Reversible and irreversible) - physostigmine, neostigmine, pyridostigmine, edrophonium chloride, tacrine hydrochloride, ambenonium chloride, isofluorphate, Echothiopate iodide, parathion, malathion.
(06) Cholinesterase reactivators – Pralidoxime chloride
(07) Cholinergic blocking agents – SAR of cholinolytic agents
(08) Solanaceous alkaloid and analogous – Atropine sulphate, hyoscyamines sulphate, scopolamine hydrobromide, homatropine hydrobromide, Ipratropium bromide.
(09) Synthetic cholinergic blocking agents – tropicamide, cyclopentolate hydrochloride, aclidinium bromide, dicyclomine hydrobromide, Glycopyrrolate, Methantheline bromide, propantheline bromide,
Cholinergic blocking agents
Anticholinergic/ Atropinic/ Parasympatholytic drugs/ Muscarinic receptor antagonists are the agents which competitively block actions of Ach on autonomic effectors and in the CNS exerted through muscarinic receptors.
Naturally occurring antimuscarinics were found in alkaloids from Belladonna (Solanaceae) plants. They were used as deadly poison and pupil-dilating cosmetics. While curare, the naturally occurring antinicotinics derived from Chondrodendron and Strychnos, was a poison used by South American Indians for hunting
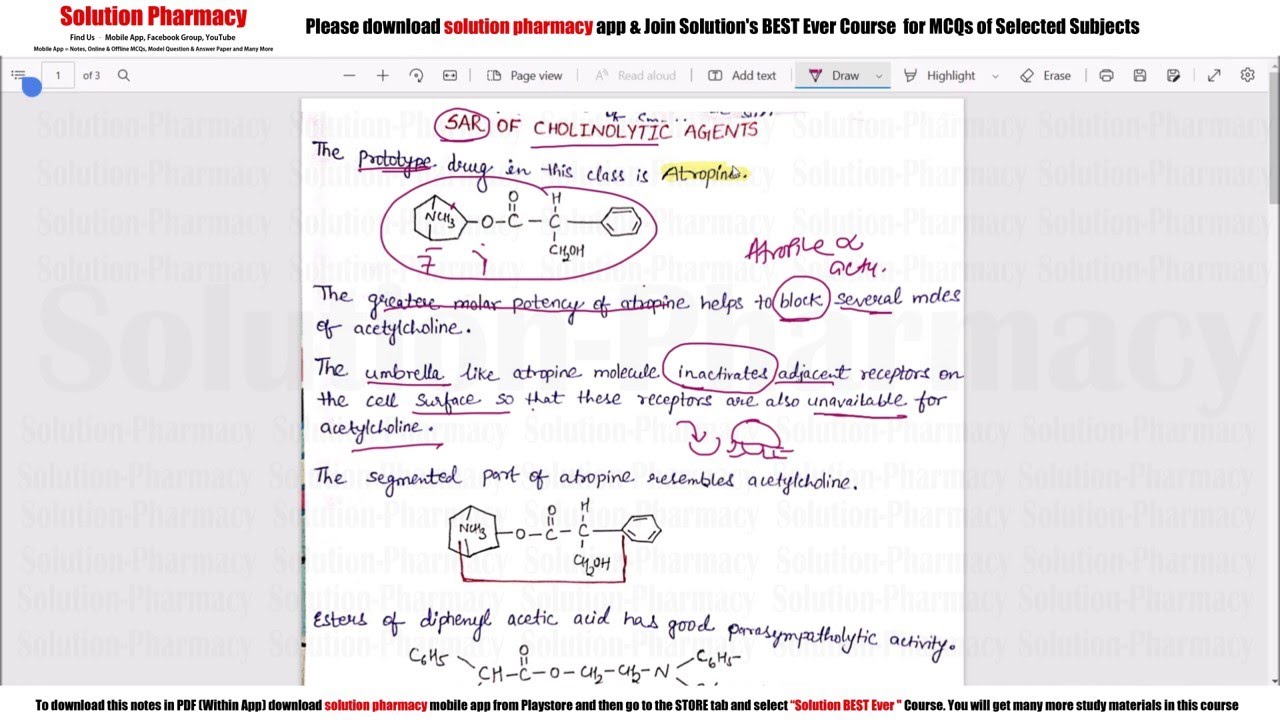
SAR of cholinolytic agents
One important structural difference between atropine and acetylcholine, both of which are esters of amino alcohols, is the size of the acyl portion of the molecules. Based on the assumption that size was a major factor in blocking action, many substituted acetic acid esters of amino alcohols were prepared and evaluated for biological activity. The most potent antagonists were those that possessed two lipophilic ring substituents on the carbon α to the carbonyl of the ester moiety.
The SAR for muscarinic antagonists can be summarized as follows:
1. Substituents R1 and R2 should be carbocyclic or heterocyclic rings for maximal antagonist potency.
2. The rings can be identical, but the more potent compounds have different rings.
3. Generally, one ring is aromatic and the other saturated or possesses only one olefinic bond.
4. Substituents R1 and R2, however, can be combined into a fused aromatic tricyclic ring system, such as that found in propantheline.
5. The R3 substituent can be a hydrogen atom, a hydroxyl group, a hydroxymethyl group, or a carboxamide, or it can be a component of one of the R1 and R2 ring systems; when this substituent is either a hydroxyl group or a hydroxymethyl group, the antagonist is usually more potent than the same compound without this group.
6. The hydroxyl group increases binding strength by participating in a hydrogen bond interaction at the receptor.
7. The X substituent in the most potent anticholinergic agents is an ester, but an ester a functional group is not an absolute necessity for muscarinic antagonist activity.
8. The N substituent is a quaternary ammonium salt in the most potent anticholinergic agents; this is not a requirement, because tertiary amines also possess antagonist activity, possibly by binding to the receptor in the cationic (conjugate acid) form.
E-Mail for official and other work - [email protected]
#solutionpharmacy #Pharmacologyclass #Pharmacognosyvideos #GPAT

Информация по комментариям в разработке