Stability and Electron Configuration
Dr. DeBacco
What Makes an Electron Configuration Stable?
An atom is most stable when its electron configuration results in:
Filled energy levels
Completely filled or half-filled subshells
Minimal electron repulsion
This stability is often achieved when atoms have a full valence shell, like the noble gases (ex. Ne, Ar), which are unreactive.
Atoms Stability
Electron configuration describes how electrons are distributed among an atom's orbitals, following the…
Aufbau principle
Pauli exclusion principle
Hund's rule
It directly influences an atom's stability, which is determined by the energy state of its electrons.
Stability generally increases when electrons occupy lower-energy orbitals or achieve specific configurations that minimize energy.
Exchange Energy
Electrons with parallel spins in degenerate orbitals (same energy) can exchange positions.
This exchange releases energy, increasing stability
Full Valence Shells (Most Stable)
A full outer level refers to a completely filled valence shell
Atoms with 8 valence electrons (or 2 for helium) are very stable because they achieve a low-energy state.
Ex. Noble gases such as He, Ne, Ar
This is the basis of the octet rule: atoms tend to gain, lose, or share electrons to achieve a full outer shell with 8 valence electrons
Except for Helium which has a full outer shell with 2 electrons.
Full Valence Shells- Examples
A full outer level corresponds to a closed-shell configuration, where all orbitals in the highest principal energy level (n) are filled, resulting in minimal energy and no need for chemical bonding.
Helium (1s²)
The 1s orbital (its valence shell) is fully occupied with 2 electrons, making helium inert.
Neon (1s² 2s² 2p⁶)
The 2s and 2p orbitals form a complete valence shell with 8 electrons, explaining neon's lack of reactivity.
Fully-Filled Subshells
A full sub-level occurs when a specific subshell (s, p, d, or f) is completely filled with electrons:
2 electrons for an s subshell
6 for p subshell
10 for d subshell
14 for f subshell
Electrons pair up in orbitals, reducing electron-electron repulsion and stabilizing the atom or ion.
Fully-Filled Subshells- Examples
Full sub-levels are energetically favorable due to their symmetry and complete pairing of electrons, often seen in stable ions or transition metals with filled d-orbitals.
Zinc (1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s²)
The 3d sub-level is fully occupied with 10 electrons, contributing to zinc's stability.
Half-Full Subshells
A half-full sub-level occurs when a subshell is exactly half-occupied with unpaired electrons:
1 electron in an s subshell (out of 2)
3 electrons in a p subshell (out of 6)
5 electrons in a d subshell (out of 10)
7 electrons in an f subshell (out of 14)
Half-full sub-levels are moderately stable
Due to exchange energy, which lowers the energy of electrons with parallel spins in orbitals with the same energy level.
This stability is less than a full sub-level but significant enough to influence electron configuration
Half-Full Subshells
The exchange energy from unpaired electrons with parallel spins reduces the overall energy, making half-filled sub-levels more stable than partially filled ones with uneven electron distribution.
Nitrogen (1s² 2s² 2p³)
The 2p subshell has 3 electrons, one in each of the three p orbitals, all with parallel spins, enhancing stability.
No Special Arrangement (Least Stable)
These atoms with no special arrangement have partially filled sub-levels with no particular symmetry and do not fit any other category…
2 or 4 electrons in a p subshell
3 or 7 electrons in a d subshell
These configurations are less stable because they lack the symmetry and energy benefits of full or half-full sub-levels.
No Special Arrangement- Examples
Partially filled sub-levels without half-full or full configurations have higher energy due to uneven electron distribution and unpaired electrons, driving chemical reactivity.
Carbon (1s² 2s² 2p²)
The 2p subshell has 2 electrons, neither half-full nor full, making carbon reactive as it seeks to form bonds to achieve an octet.
Stability and Reactivity
Stable configurations → low reactivity (ex. noble gases)
Unstable configurations → high reactivity (ex. alkali metals, halogens)
Atoms will gain, lose, or share electrons to reach a more stable configuration
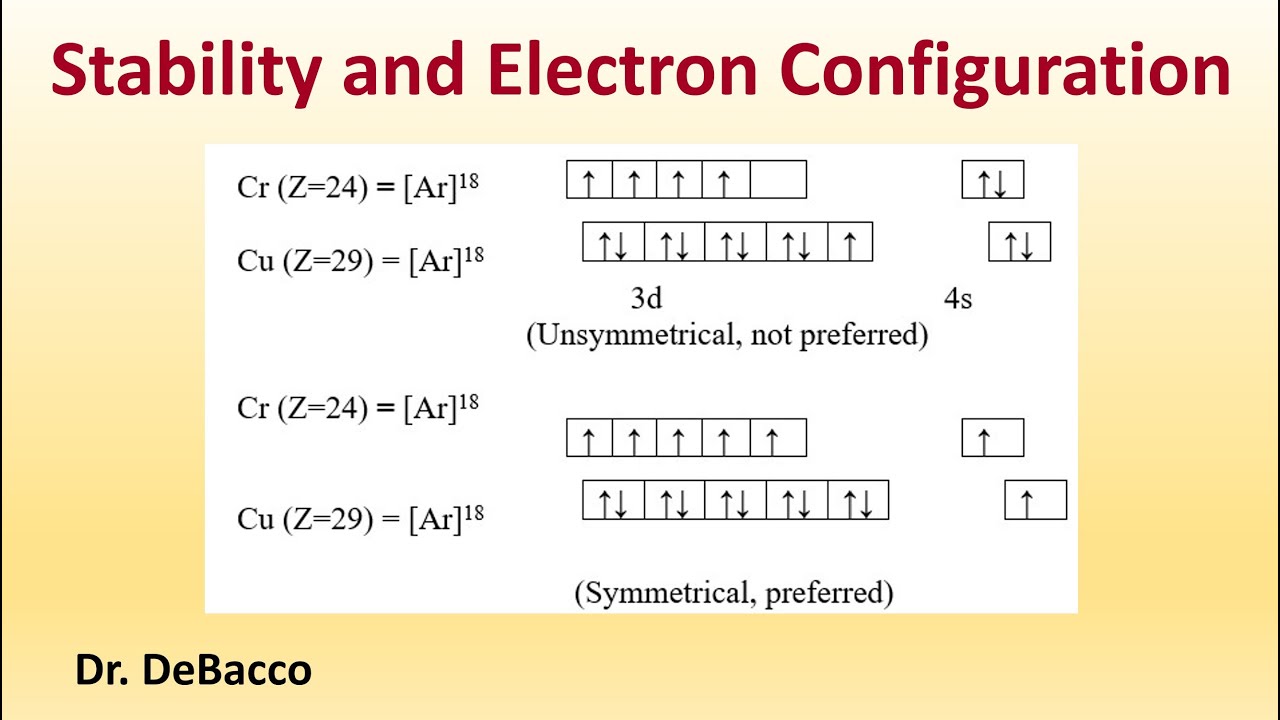
Exceptions
Transition metals like chromium and copper deviate from the Aufbau principle because half-full or full d sub-levels are more stable.
Example
Cr: 3d⁵ 4s¹ instead of 3d⁴ 4s²
Link to Lecture Slides: https://drive.google.com/file/d/12EnH...
*Due to the description character limit the full work cited for "Stability and Electron Configuration" can be viewed at... https://docs.google.com/document/d/1r...

Информация по комментариям в разработке