What titration curves look like and how to read them for AP Chemistry.
Stoichiometry Titration Problem Video: • Stoichiometry Problem: Titration Calc...
TRANSCRIPT:
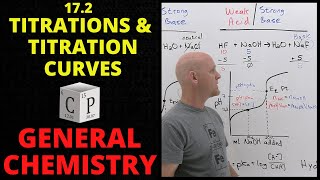
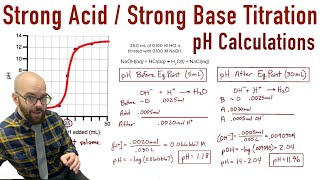
In the simplest terms, a titration curve is basically a plot of the volume of titrant that you’re adding to the analyte versus pH. What the general shape of a titration curve looks like is kind of like this, and if you haven’t seen my titration stoichiometry problem video yet, I’d highly recommend that you look at that. Back to this, this is a curve of a strong acid and it is being titrated with a strong base. And you can tell that it’s starting out with a strong acid because the pH is very low. And remember that the lower the pH is, the stronger the acid is. So here we’re going to add more and more titrant, or more and more of the strong base, and here you see a big jump up to a very high pH so over here it’s very basic and here it’s very acidic. Here is what we call the equivalence point – it’s pretty much halfway up this huge jump. And what’s happening is we have our acid, so let’s say HCl because that’s a strong acid, and when you have it in solution, it’s present as H+ ions and Cl- ions. But when you add a strong base to it, let’s say NaOH, that goes into solution as Na+ and OH- ions. Now here, the Na+ and Cl- are spectator ions because they don’t really do anything or change chemically; it’s just that these H+ and OH- ions are going to combine to neutralize each other and make H2O. So what’s happening here is we have a lot of HCl and not that much NaOH yet. Because we have a lot of HCl, which is a strong acid, that’s causing the solution to be very acidic. Now here, the equivalence point is where the moles of HCl equal the moles of NaOH. As you can see, the equivalence point is at a pH of around 7, which is perfect because we have a strong acid and a strong base. When they have equal moles, the pH is 7 or neutral. Up here, we have a lot of NaOH so now NaOH is in excess and it’s causing the solution to be basic. But let’s see what happens when we have a strong acid and a weak base.
Here you see that the pH is still really low, meaning that we have our strong acid to start with. So let’s go with HCl again. And for our weak base, let’s go with NH3 which is ammonia, and we see that our equivalence point is around here. Now notice that this isn’t at 7 like the other titration curve that we looked at. It’s a little below 7, so that means that at the equivalence point, the solution is slightly acidic. And the reason for that is because we have a weak base instead of a strong one that’s balancing out the effects of the strong acid. And also, if we write this out, the equation for NH3 when it’s placed in water is, it partially ionizes into NH4+ and OH-. Since this doesn’t go to completion, some of it stays as NH3 and some of it changes into NH4+. Since NH3 is a weak base, its conjugate acid, NH4+, is a little stronger than the strength of NH3. So since this is a stronger acid, it’s also causing the solution to be a little acidic. And you’ll see something similar to that when we look at the weak acid and strong base titration curve.
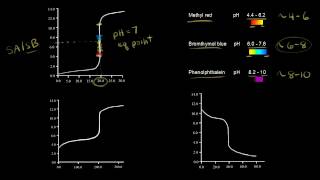
So we’ve got the same general shape for a titration curve with a weak acid and a strong base, but notice that the pH isn’t as low as what it was before. Before it was around here. Because we’re starting out with a weak acid, the pH isn’t going to be as low. Plus, when we look at the equivalence point, let’s say it’s around here, and move over to where it says the pH is, the pH is a little higher than 7. That means it’s going to be a little basic. Following the logic that we used for the strong acid-weak base titration curve, when we have a weak acid such as acetic acid, that partially dissociates into acetate and H+ ions. But remember, it’s only partial dissociation. So, because acetic acid is a weak acid, its conjugate base is stronger. Since acetate is more of a strong base, it’s going to accept protons and change back into this. So it’s going to cause the pH at the equivalence point to be a little higher than 7. Also, I’d like to point out that in here, you have what is called a buffer region. What’s happening here, is when we titrate the weak acid with a strong base, we have a conjugate acid-base pair. So when we have a strong base such as NaOH, it’s going to steal the H+ ions from C2H3O2. So what that’s going to create is a bunch of acetic acid and acetate. That’s going to create a buffer because there are a bunch of conjugate acids and bases. In the middle of the buffer region which is half of the equivalence point, we’re actually going to see that the pH is equal to pKa. And remember from the Henderson-Hasselbalch equation which is pH = pKa + log([A-]/[HA]) that whenever these two are the same, that just simplifies to 1, and the log of 1 is 0. So what we get from here is that pH = pKa meaning that the concentrations of the conjugate base and the conjugate acid are the same.






Информация по комментариям в разработке