00:00 Introduction and ionizing the vaporized sample.
01:06 Ions pass through the accelerating potential and the beam is sharpened.
01:54 The beam passes through a velocity selector (Wien filter) to precisely tune the speed.
03:27 Particles are deflected in the analyzer region (magnetic sector).
05:23 How to search for particles over a large mass range.
06:43 The mass spectrum of a sample.
07:37 Example of computing mass to charge ratio and mass, and identifying an element.
New videos every week! Subscribe to Zak's Lab / @zakslab
Measuring mass to charge ratio with a mass spectrometer:
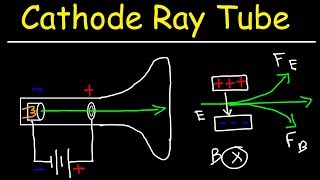
A magnetic sector analyzer allows us to find the mass of ionized atoms and molecules by taking advantage of the fact that the curvature of a charged particle in a magnetic field depends on the mass of the ions deflection in a magnetic field.
We start with a vaporized sample pumped into the ionizing chamber. We hit the gas atoms with energetic electrons, and the collisions knock an electron off the atoms or molecules, leaving these particles positively charged. The ionization process usually produces a single ionization, so the resulting charge on the ionized particle is +q_e. Occasionally we get double ionizations, resulting in a charge of +2q_e. An ion repeller pushes the ions out of the ionization chamber past a positive plate, where the ions are accelerated to high speed through a potential difference.
As the ions cross the accelerating potential, they pass through more slits in order to narrow the beam. Ions crashing into the plates will pick up an electron and be neutralized.
At the next stage, we have a Wien filter or velocity sector consisting of crossed electric and magnetic fields. The purpose of the Wien filter is to create a very narrow band of velocities in the beam, and this improves the resolution of the spectrometer, reducing the error bars on mass to charge calculations. This is necessary because, although all the particles have been accelerated through the same potential difference, even particles with equal mass emerge with a distribution of speeds from the ionizing chamber, just due to their random motion in the gas.
Next, the beam enters the analyzer region, in which we see an outward pointing magnetic field. The magnetic force does no work because it’s perpendicular to the velocity, but it does turn the velocity vector. The magnetic force is pointing to the center of curvature, and we have uniform circular motion for the particles in the beam.
We use Newton’s second law and the formula for centripetal acceleration to relate the charge and mass to the radius of curvature, strength of the magnetic field and speed of the particles. This allows us to calculate the mass to charge ratio for the particles.
Finally, the particles arrive at the detector. This is a stream of positively charged ions, so we are measuring a current here!
How do we find other atoms and molecules? If we’re dealing with a heavier atom, it will emerge moving slower from the accelerating potential and therefore get screened off by the speed selector. Furthermore, two isotopes making it through the speed selector might have masses that are very nearly the same, but maybe the lighter one is detected, while the heavier one moves with a larger radius of curvature and misses the detector.
The resolution to these questions is that we have control over the knobs in this experiment! Depending on the design of the mass spectrometer, we may be able to vary the accelerating potential, the speed selector and the magnetic field in the analyzer region.
We can imagine gradually increasing the magnetic field in the analyzer region, and detecting that slightly heavier isotope as we sweep upward in magnetic field strength.
In practice, a mass spectrometer will sweep through a wide range of settings to detect particles with many different masses. We can then display the contents of the sample in a mass spectrum showing the relative abundance of each particle as a function of m/z (mass to charge ratio).
Note that a spectrum may contain one line for a singly ionized atom and another for its doubly ionized counterpart. We can infer we are probably looking at two ionizations of the same atom.
In addition, when we are studying molecules, the molecules can be fragmented in the ionization process. Thus, a single molecule can produce many lines corresponding to the masses of all the fragments. This is actually useful because it gives us a characteristic “fingerprint” for identifying the molecule. In fact, in the spectrum of toluene shown in the video, that’s exactly what we’re looking at.
Finally, we wrap things up by computing the mass to charge ratio for a beam, converting the units to atomic mass units over q_e, then getting the mass of the particles assuming they are singly ionized. We check against the periodic table and discover that our beam is made of Carbon 12 ions.




![Звуковые иллюзии, которые работают на всех (почти) [Veritasium]](https://i.ytimg.com/vi/8pCuUfbdheE/mqdefault.jpg)




Информация по комментариям в разработке