This Harvard Medical School Continuing Education video examines these key questions: what are CAR T cells, in what diseases are they utilized, and what are the common toxicities of CAR T cells?
Dr. Connor Johnson, MD, an oncologist at Massachusetts General Hospital, discusses chimeric antigen receptor cell therapy (CAR T) with a particular focus on lymphoma. The logistics of CAR T-cell therapy are described and toxicities are identified, including cytokine release syndrome (CRS), ICANS, and cytopenias.
00:00 | Introduction
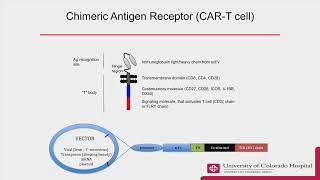
00:37 | Overview of CAR T cells and immunology principles
02:16 | CAR T cells therapies and targets
02:48 | Logistics of CAR T-cell therapy
03:43 | Toxicities related to CAR T-cell therapy
This video was peer reviewed by Dr. Jonathan Salik, TMD, MHPEd, Instructor of Medicine, Massachusetts General Hospital; and Dr. Sugantha Sundar, MD, Assistant Professor of Anesthesia, Beth Israel Deaconess Medical Center, to validate the quality and accuracy of the content. It was edited by affiliate physicians of Harvard Medical School, Arielle J Medford, MD, Senior Medical Oncology Fellow, Dana-Farber Cancer Institute and Anna Handorf, MD, Research Fellow in Pediatrics, Massachusetts General Hospital.
References:
Gaudino SJ and Kumar P. Cross-Talk Between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front Immunol. 2019 Mar 6;10:360.
Kawalekar OU, O'Connor RS, Fraietta JA, et al. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity. 2016;44(2):380-390. doi:10.1016/j.immuni.2016.01.021
Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo [published correction appears in Mol Ther. 2015 Jul;23(7):1278]. Mol Ther. 2009;17(8):1453-1464. doi:10.1038/mt.2009.83
Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531-2544. doi:10.1056/NEJMoa1707447
Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380(1):45-56. doi:10.1056/NEJMoa1804980
Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839-852. doi:10.1016/S0140-6736(20)31366-0
Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med. 2020;382(14):1331-1342. doi:10.1056/NEJMoa1914347
Jacobson CA, Chavez JC, Sehgal AR, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23(1):91-103. doi:10.1016/S1470-2045(21)00591-X
Munshi NC, Anderson LD Jr, Shah N, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N Engl J Med. 2021;384(8):705-716. doi:10.1056/NEJMoa2024850
Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314-324. doi:10.1016/S0140-6736(21)00933-8
Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491-502. doi:10.1016/S0140-6736(21)01222-8
Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439-448. doi:10.1056/NEJMoa1709866
Hartmann J et al. Clinical development of CAR T-cells—challenges and opportunities in translating innovative treatment. EMBO Mol Med. 2017 Sep;9(9):1183-1197.
Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. Published 2018 Jun 15. doi:10.1186/s40425-018-0343-9
Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25(4):625-638. doi:10.1016/j.bbmt.2018.12.758
Notice: At this time, the content in this video is not accredited.







Информация по комментариям в разработке