Need help preparing for the Bio/Bio Chemistry section of the MCAT? MedSchoolCoach expert, Ken Tao, will teach everything you need to know about Michaelis-Menten Kinetics which is a key component of the biochemistry section of the MCAT. Watch this video to get all the MCAT study tips you need to do well on this section of the exam!
Michaelis-Menten kinetics is a model of enzyme kinetics developed by the German biochemist Leonor Michaelis and Canadian physician Maud Menten. By relating the rate of an enzymatic reaction to the substrate concentration, this model can characterize several types of reactions.
Enzymes
In order to understand the concept of Michaelis-Menten kinetics, it is important to review the basic function of enzymes. Enzymes are biological catalysts within cells that are generally made of proteins, but sometimes they are made of RNA molecules, we well. As catalysts, there are several features of enzymes that are important to understand. First, enzymes increase the rate of the reaction by decreasing the activation energy. Enzymes accomplish this by decreasing the activation energy of the reaction. Second, there is no net consumption or production of enzymes during a reaction. In this way, it is said that enzymes are recycled during a chemical reaction. Lastly, enzymes are reaction-specific. For example, in glycolysis, the first step of the reaction is catalyzed by the enzyme hexokinase, which converts glucose into glucose-6-phosphate. Hexokinase, however, is not able to catalyze any of the other steps in glycolysis. Thus, hexokinase is specific to this reaction.
Michaelis-Menten Kinetics Model
The Michaelis-Menten kinetics model describes the general catalysis of enzymatic reactions. Figure 1 shows an equation that represents the basic concept of this model. The enzyme (E) binds to the substrate (S) to form the enzyme-substrate complex (ES). The enzyme-substrate complex (ES) can then react to form the enzyme and the product.
Other essential features of this equation include the variables k1, k2, and k3. Recall from General Chemistry, that lowercase k stands for the rate constant, which is a description of the speed of a reaction. In this model, k2 can also be understood as kON, k1 as kOFF, and k3 as kCAT. In this way, Kon (k2) is how fast the enzyme binds to the substrate to form the enzyme-substrate complex (ES). Likewise, kOFF (k1) is how fast the enzyme-substrate complex (ES) dissociates to form the free enzyme (E) and substrate (S). Finally, kCAT (k3) refers to how fast the enzyme-substrate complex (ES) forms that enzyme (E) and product (P). Also, it is important to note that the first step in this equation is reversible, meaning it can go in the forward or reverse direction. However, the second step can only go in the forward direction, so it is irreversible.
Michaelis-Menten Equation
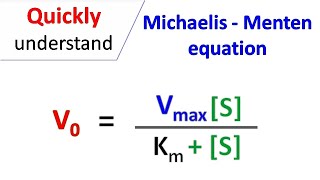
From the Michaelis-Menten model, scientists derived the following equation:
v = (Vmax) x [S] / Km + [S]
This equation is describing v or the reaction velocity. The reaction velocity (v) is how fast products are being formed. Vmax describes the maximum reaction velocity, and [S] is the substrate concentration. The variable Km is the Michaelis constant. It is equal to the substrate concentration when the reaction velocity is equal to half the maximum reaction velocity, or half Vmax. The Km value is dependent on several factors, like the specific enzyme and substrate, the temperature of the reaction, as well as the pH. Also, in equation form Km = kOFF + kCAT / kON. Overall, the Michaelis-Menten equation describes how fast a reaction is compared to the concentration of substrate and solution.
Michaelis-Menten Assumptions
For the MCAT exam, it is important to understand certain key assumptions that were made in deriving the Michaelis-Menten equation. In this way, it is possible to discern when the equation can be used and when it cannot be used. The first assumption is that this equation is only used to describe the initial reaction velocity. Even though the variable v is the reaction velocity, in the equation, it is only the initial reaction velocity. One of the consequences of this assumption is that the substrate concentration is much greater than the product concentration. In the initial stage of the reaction, not much product is formed, so this assumption makes sense.
MEDSCHOOLCOACH
To watch more MCAT video tutorials like this and have access to study scheduling, progress tracking, flashcard and question bank, download MCAT Prep by MedSchoolCoach
IOS Link: https://play.google.com/store/apps/de...
Apple Link: https://apps.apple.com/us/app/mcat-pr...
#medschoolcoach #MCATprep #MCATstudytools






![Enzyme Kinetics with Michaelis-Menten Curve | V, [s], Vmax, and Km Relationships](https://i.ytimg.com/vi/kmyR1cYxRL4/mqdefault.jpg)

Информация по комментариям в разработке