Need help preparing for the Bio/Bio Chemistry section of the MCAT? MedSchoolCoach expert, Ken Tao, will teach everything you need to know about Enzyme Inhibition of Michaelis-Menten Kinetics which is a key component of the biochemistry section of the MCAT. Watch this video to get all the MCAT study tips you need to do well on this section of the exam!
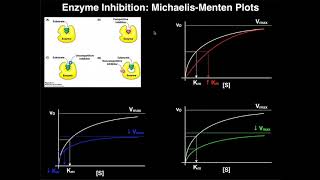
An important concept of Michaelis-Menten Kinetics is enzyme inhibition. Enzyme inhibitors will bind to different parts of this equation in order to prevent the reaction from taking place. Also, remember that the Michaelis-Menten saturation curve represents the rate of an enzymatic reaction compared to the substrate concentration in the reaction. Different enzyme inhibitors can affect this curve in different ways, all of which are important to understand for the MCAT exam.
Competitive Inhibition
In competitive inhibition, the inhibitor binds directly to the enzyme to form an enzyme-inhibitor complex. This enzyme-inhibitor complex cannot react, so when it forms, the enzyme will be unable to produce the product. In this way, the reaction is stopped from moving forward. Moreover, it is crucial to understand where an enzyme will bind to an inhibitor. In competitive inhibition, the inhibitor is competing directly with the substrate to bind to the enzyme. In normal circumstances, the substrate binds to the enzyme's active site. Similarly, competitive inhibitors also bind to the enzyme's active site.
Competitive inhibition also affects Km and Vmax. Recall that the Km is inversely related to the affinity of the enzyme for the substrate. In this way, when an inhibitor is added, it makes it harder for the enzyme to bind to its substrate. Although the enzyme wants to bind to the substrate, the inhibitor gets in the way. In this way, in the presence of competitive inhibition, the affinity of the enzyme for the substrate decreases. Similarly, the value of Km increases.
The question for any inhibition in terms of this curve is at extremely high substrate concentrations, is the inhibitor going to affect the maximum reaction velocity? In terms of competitive inhibition, the answer is no. It does not affect Vmax. When there is more substrate then inhibitor present, the substrate will outcompete the inhibitor for the active site of the enzyme. Therefore, increasing the substrate concentration is a way to overcome competitive inhibition and reach the maximum target velocity. However, what changes is the substrate concentration needed to reach the maximum velocity in the presence of a competitive inhibitor. With a competitive inhibitor present, the amount of substrate needed to reach Vmax is much greater than without the inhibitor.
Uncompetitive Inhibition
Another type of enzyme inhibition is known as uncompetitive enzyme inhibition. In uncompetitive inhibition, the inhibitor does not directly compete with the substrate to bind the enzyme. The uncompetitive inhibitor cannot even bind directly to the enzyme itself. Instead, it can only bind to the enzyme-substrate complex [ES]. When it binds, it forms an enzyme-substrate-inhibitor complex, which, like in competitive inhibition, cannot react, and the reaction, therefore, is unable to proceed. Also, the uncompetitive inhibitor, unlike the competitive inhibitor, does not bind to the enzyme's active site. It binds to a site other than the active site. In scientific terms, this site is known as the allosteric site.
In terms of Km, recall that it is inversely related to the affinity of the enzyme for the substrate. When an uncompetitive inhibitor is added, the enzyme-substrate complex will still form with the inhibitor bound to it. In this way, the concentration of enzyme [E] and substrate [S] are being used up in the reaction and will decrease overall. Therefore, the whole Michaelis-Menten saturation curve will shift to the right, which will result in more enzyme binding to the substrate. In other words, the inhibitor artificially increases the affinity of the enzyme for the substrate by combining the two with the inhibitor. As a result, then, the enzyme's affinity for the substrate increases, which means the Km is going to decrease.
In terms of the Vmax, unlike in competitive inhibition, using extremely high substrate concentrations is unable to overcome an uncompetitive inhibitor. Adding more substrate to the reaction will result in the formation of more enzyme-substrate complexes. The enzyme-substrate complex is precisely what an uncompetitive inhibitor wants to bind. Therefore, in uncompetitive inhibition, the Vmax decreases.
IOS Link:
https://play.google.com/store/apps/de...
Apple Link:
https://apps.apple.com/us/app/mcat-pr...
#medschoolcoach #MCATprep #MCATstudytools









Информация по комментариям в разработке