Learn about Arrhenius and Broasted-Lowry acid-base theory, the amphoteric nature of water and some properties of acids and bases in this video!
transcript
_______________________________________
every day you encounter acids and bases. you probably ate food with acids in it and then washed the dishes with a base. There are some basic properties that will help you distinguish acids and bases from other chemicals.
Acids have a sour or tart taste. Think of lemons, or vinegar, that’s the taste. bases taste bitter, which is not a pleasant taste. if you’ve ever had soap in your mouth, you’ll know that bitter taste. Both acids and bases will change the color of an acid-base indicator, they both can be strong or weak electrolytes in solution and they are both corrosive. Always check the safety when working with acids and bases to make sure you protect yourself and your clothing.
Acids also have a slightly different naming convention compared to other chemicals. Here are some common acid names that you’ll come across in the chemistry lab.
it wasn’t until the late 1800s that a scientist was able to propose a theory for what makes an acid and acid and a base, a base. That scientist was Svante Arrhenius. He said that acids are hydrogen containing compounds that ionize to create hydrogen ions (or hydronium) in solution and bases are compounds that ionize to create hydroxide ions in solution. Let’s look at an example of this happening.
Hydrochloric Acid is a hydrogen atom and a chlorine atom covalently bonded. In water, the hydrogen abandons the chlorine, leaving its electron behind and then it joins the water molecule to form hydronium ion. Because hydrogen can be removed as an ion, hydrochloric acid is ionizable.
hydrochloric acid releases one hydrogen so it’s called monoprotic. some acids have multiple hydrogen atoms that will ionize and could be diprotic if they release two hydrogen ions like sulfuric acid or or triprotic if they release three like phosphoric acid.
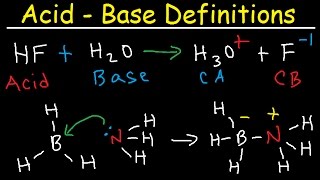
let’s look again at hydrochloric acid in water. Hydrochloric acid is an arrhenius acid because it creates a hydrogen ion. the hydrogen ion immediately attached itself to water to form hydronium, H3O+ which is the aqueous form of hydrogen ion.
arrhenius bases produce hydroxide, so it’s no surprise that all of these bases have hydroxide in their names. potassium hydroxide and sodium hydroxide are strong bases and are very soluble in water. calcium hydroxide and magnesium hydroxide are strong bases but don’t dissolve easily in water which makes the solution very diluted with hydroxide ions.
the arrhenius definition, it turns out, is too confining for all acids and bases. for example, ammonia is basic, but there is no hydroxide on ammonia. in the presence of water, however, it will react to produce hydroxide from the water molecule. So there is another way to define acids and bases.The Bronsted-Lowry definition is that acids are hydrogen ion donors and bases are hydrogen ions acceptors. It’s a broader definition.
all arrhenius acids and bases are also bronsted-lowry acids and bases, but not all bronsted-lowry acids and bases are arrhenius.
when you look at an acid base reaction, you’ll notice that a double arrow is often used. this implies that the reaction is actually reversible, it can occur in both directions. In this reaction, the ammonia is the base, because it is the hydrogen acceptor. by default then, the water is acting as an acid because it will the the hydrogen donor. but when we look at the other side of the equation, we could imagine it going in the opposite direction where ammonium ion would donate the proton and hydroxide would accept. we call these the conjugate acid and conjugate base. A conjugate acid is the particle formed when a base gains a hydrogen ion. A conjugate base is the particle that remains when an acid has donated a hydrogen ion. They will always be paired like this because this is a reversible reaction.
in this scenario, the hydrochloric acid has a conjugate base of chloride ion, and water is the base to hydronium as the conjugate acid. With ammonia, however, water acted as an acid.
water is incredibly special for many reasons, and one of those is that it’s amphoteric. it’s able to act as both an acid AND a base! if you break off one hydrogen atom, you get a hydrogen ion and a hydroxide ion. the hydrogen ion would imply that water is an acid, and the hydroxide would imply that water is a base. the truth is, it’s both! Amazing!









Информация по комментариям в разработке