Often in biochemistry we do various sorts of “fractionation” where we take a solution containing a mix of molecules and separate those molecules into different parts or “fractions” based on various properties (size, charge, etc.). We frequently separate molecules within a mixture with techniques like column chromatography where we send the mixture through a column filled with little beads (resin) with various properties. The molecules in the solution have different properties, so they’ll interact differently with the resin, causing them to travel at different speeds through the column, thus separating from one another. If we capture them as they come out of the column, we have a chance to isolate the different types of molecules in a purer form. I say we have a chance, because it’s not a sure bet. If the molecules are too similar with regards to what the separation is based on, they won’t separate enough to isolate them individually. But even if they do separate enough, you need to capture each one separately before the other one comes out! And this is where fraction collection comes in.
We basically choose a fraction size (e.g 1 mL) and then as the liquid comes out we collect small portions (fractions) of that size. So we’d collect the first mL to come out in the first tube, 2nd ml in the second, etc. It’s like the molecules are coming out of a building and filling up a line of buses, first-come first-serve.
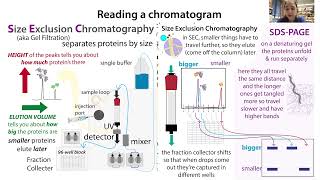
We can do this with automated fraction collectors or manually (e.g holding a tube under the stopcock and quickly shifting it to another tube once the first tube is filled to your liking). Typically, automated fraction collectors, such as those on an AKTA or an HPLC machine, are hooked up to a UV detector to monitor the absorption of the solution as it comes out. Molecules like proteins and nucleic acids absorb UV light so if they’re present in the solution we’ll be able to see evidence of them. The more of them there are, the stronger the signal. And this will correspond to a peak on a chromatogram - the readout you get as the molecules come out, pass the UV detector, and go into the tube/well, etc.
much more on working with chromatograms here: https://bit.ly/chromatograms & • Interpreting & working with protein c...
If you’re fractionating manually, you can measure each fraction separately with a spectrophotometer or other method (such as a Bradford assay to measure protein concentration).
Depending on the properties of the molecules and your separation method, some of the molecules will end up overlapping in where they come out so you’ll have to decide which fractions have what you want and don’t have too much of what you don’t what - which are good enough will depend on your goals.
If you care most about quantity (yield), be less stringent in which fractions you pool. Include the shoulders, even if they’re bumpy, etc.
If you care most about purity, be more stringent in which fractions you pool. Run tests (e.g. gel electrophoresis) to see which fractions have acceptable purity and only combine those. And keep your samples on ice in the meantime when appropriate.
In order to have the best chance at being able to collect one component without the other, collect smaller fractions. But you don’t want to collect too small of fractions because that’s just a pain to combine! Plus you have more chances to accidentally combine the wrong fractions. And the more spread out the sample is and the more liquid transfers you have to do, the more opportunities there are to lose yield because of tiny bits of liquid left stuck in the tube, tip, well, etc.
Sometimes, UV or Bradford doesn’t tell you enough. That only tells you about total protein etc. not whether the one you actually care about is in there! So you might want/need to run a gel, do a western, or even do an enzymatic activity assay in order to choose which ones to pool and keep. In the meantime, keep your samples on ice (if we’re talking about a protein, RNA, or anything else that should be kept cold.
Notes of caution:
* sometimes, especially if the tubing is too long, the actual fractions don’t correspond exactly to how they appear on the graph. So be cautious and maybe run a gel or something for edge cases.
* Also be cautious of the fractionator missing the tubes - you might need to manually
* even if the fractionator makes it into the tube, there might not be enough in the tube - if you see that the fraction volumes are uneven, that could indicate air in the system (you might need to primer the AKTA pump for example)
links to related posts in comments









Информация по комментариям в разработке